The atomic mass unit is the system of measurement designed to identify each individual unit of mass in atoms and molecules. Also known as a dalton, the atomic mass unit is a universally-applied measurement based on 1/12 the total mass of a single carbon-12 atom. This means that a carbon-12 atom has the atomic mass of 12 daltons. The designation for a standard atomic mass unit is u or Da. Atomic mass units are used as the system of measurement in every science, except for those involving biology and biochemistry, which use the dalton designation.
Amu Chemistry Pronunciation
One convenient aspect of atomic mass units is that, while based on carbon mass, a single unit is also equal to one hydrogen atom. This is because the combined mass of a single proton and neutron, the composition of a hydrogen atom, is equal to the measurement. Electrons, being only 1/1836 the mass of a proton, are essentially negligible to the overall mass of an atom.

Build a solid foundation in biology, chemistry, physics, mathematics, and earth science with an online Bachelor of Science (BS) in Natural Sciences from American Military University (AMU). An amu is a unit of mass, like a pound, gram, microgram, or tonne. An amu is a very, very small unit of mass, defined, as you know by now, as being exactly the mass of 12 isolated 12 C atoms in their ground state (which is just a way to eliminate kinetic or potential energy which would give the atoms more mass (remember E = mc²)).
One of the most problematic aspects to using the atomic unit of mass to define atoms is that it does not account for the energy that binds together an atom's nucleus. Unfortunately, this is not a fixed mass due to the differences between each different types of atom. As more protons, neutrons and electrons are added to an atom to create a new element, the mass of this binding energy changes. This means that the measurement can be said to be a rough approximation rather than an exact constant.
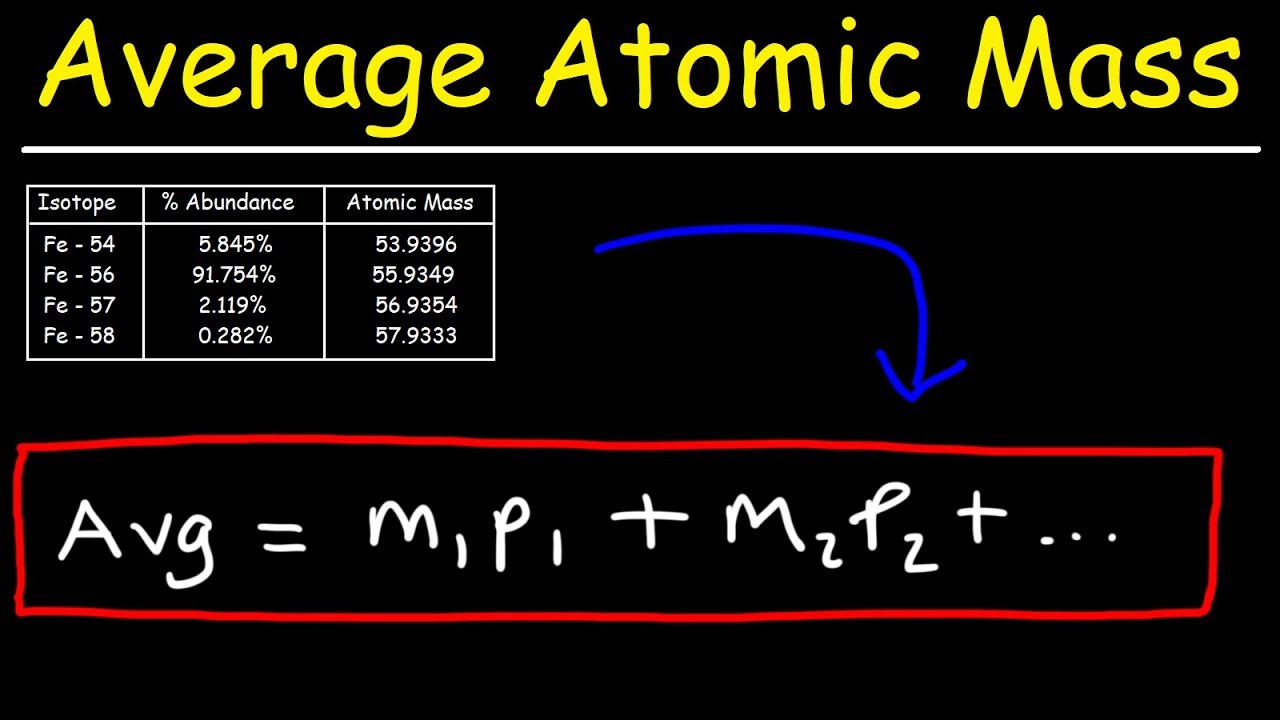
The atomic mass of chlorine is 35.45 amu. Calculate the atomic mass of Nickel using the information provided in the following table. Answer: To find the atomic mass of nickel, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form) and then the individual masses are added together. American Military University. Congress Street Charles Town, WV 25414. Help Center; Admissions FAQ; Tuition & Financing FAQ; Financial.
One of the main uses for the atomic mass unit involves its relationship with moles. Dr web cureit for mac os. A mole is the complete physical quantity of a single unit of a substance. For example, a single water molecule, comprised of two hydrogen atoms and a single oxygen atom, is a mole of water. This means that it has the atomic mass of all three atoms.
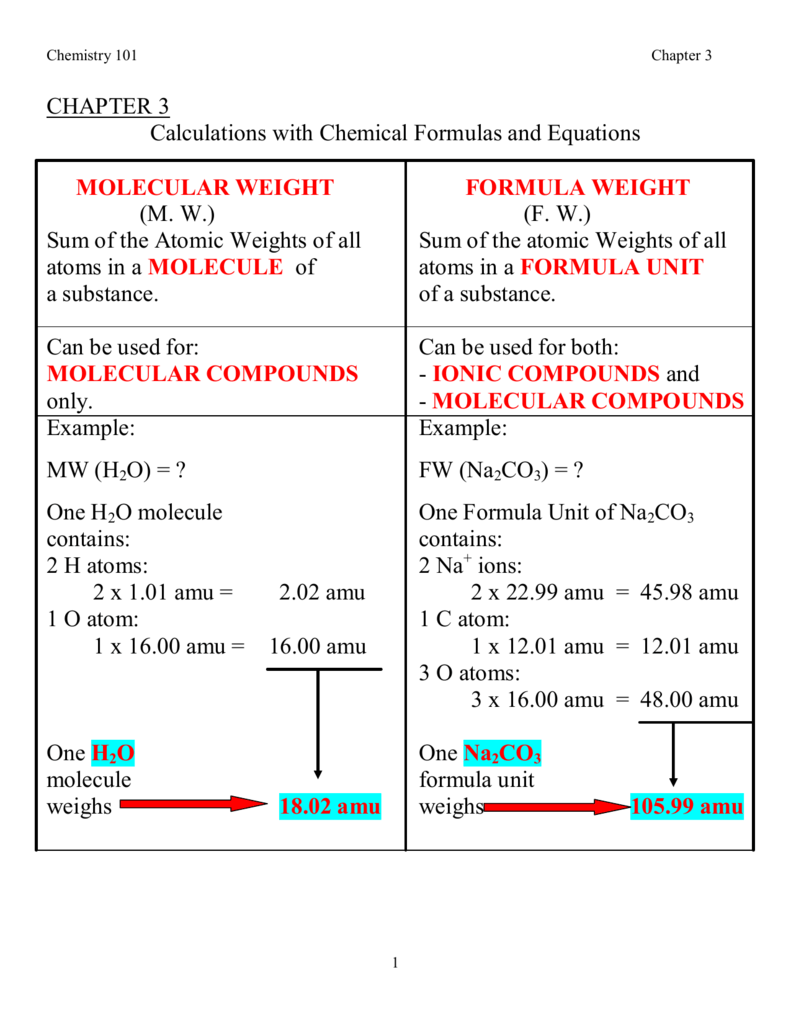
The establishment of the atomic mass unit was first started by a chemist name John Dalton in the early 1800s. He used a single hydrogen atom as the platform for the measurement. However, this was altered by Francis Aston with his invention of the mass spectrometer in the late 1800s. Aston defined an atomic mass unit as being 1/16 the mass of a single oxygen-16 atom. It wasn't until 1961 that the International Union of Pure and Applied Chemistry defined the modern applications of the measurement and linked it to carbon-12.
Amu Chemistry Conversion


Amu Chemistry Carbon
The best video editing software for mac. The Chemistry Department is one of the oldest departments in the University with the tradition of high quality research and teaching in Analytical, Inorganic, Organic and Physical Chemistry. It was given a status of a Department in 1911 under the Chairmanship of Prof. H.B. Duncliff. It was the time when M.Sc. teaching also commenced. The foundation of the present building was laid in 1926 and since then it has been serving undergraduate, postgraduate, and research students. Industrial Chemistry course was introduced in the Department at undergraduate level in 1994-1995 and at postgraduate level in 1999-2000. The Chemistry Department has a pivotal position in the area for its leadership role in training for research and has provided support by way of access to its seminar library, research journals, analytical facilities, instrumentation support and guidance. The Department was awarded SAP (DRS -1) programme in 2009 and FIST programme in 2011. The SAP (DRS -II) has been awarded in 2014 amount of 1.5 Crore..
