- There are 47 electronsin a neutral silver atom. Silver has an atomic number of 47, which means it has 47 protons in its nucleus. A neutral atom of silver will have an equal number of electrons.
- Its 47 electrons are arranged in the configuration Kr4d 10 5s 1, similarly to copper (Ar3d 10 4s 1) and gold (Xe4f 14 5d 10 6s 1); group 11 is one of the few groups in the d-block which has a completely consistent set of electron configurations.
There are 47 electrons in a neutral silver atom. Silver has an atomic number of 47, which means it has 47 protons in its nucleus. A neutral atom of silver will have an equal number of electrons.
Element Silver - Ag
Comprehensive data on the chemical element Silver is provided on this page; including scores of properties, element names in many languages, most known nuclides of Silver. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Silver Menu
- Silver Page One
- Silver Page Two
- Silver Page Three
Overview of Silver
- Atomic Number: 47
- Group: 11
- Period: 5
- Series: Transition Metals
Silver's Name in Other Languages
- Latin: Argentum
- Czech: Stříbro
- Croatian: Srebro
- French: Argent
- German: Silber - s
- Italian: Argento
- Norwegian: Sølv
- Portuguese: Prata
- Russian: Серебро
- Spanish: Plata
- Swedish: Silver
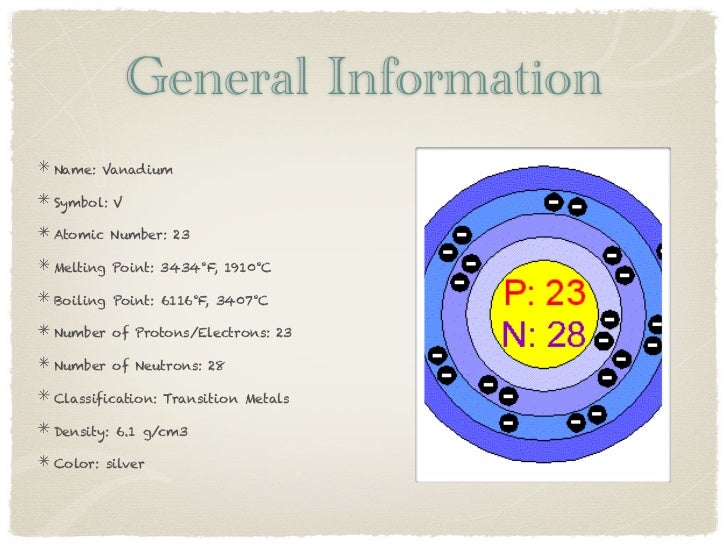
Atomic Structure of Silver
- Atomic Radius: 1.75Å
- Atomic Volume: 10.3cm3/mol
- Covalent Radius: 1.34Å
- Cross Section (Thermal Neutron Capture)σa/barns: 63.6
- Crystal Structure: Cubic face centered
- Electron Configuration:
- 1s2 2s2p6 3s2p6d10 4s2p6d10 5s1
- Electrons per Energy Level: 2,8,18,18,1
- Shell Model
- Shell Model
- Ionic Radius: 1.26Å
- Filling Orbital: 4d10
- Number of Electrons (with no charge): 47
- Number of Neutrons (most common/stable nuclide): 61
- Number of Protons: 47
- Oxidation States: 1
- Valence Electrons: 4d10 5s1
Chemical Properties of Silver
- Electrochemical Equivalent: 4.0246g/amp-hr
- Electron Work Function: 4.26eV
- Electronegativity: 1.93 (Pauling); 1.42 (Allrod Rochow)
- Heat of Fusion: 11.3kJ/mol
- Incompatibilities:
- Acetylene, ammonia, hydrogen peroxide, bromoazide, chlorine trifluoride, ethyleneimine, oxalic acid, tartaric acid
- Ionization Potential
- First: 7.576
- Second: 21.49
- Third: 34.83
- Valence Electron Potential (-eV): 11.4

Physical Properties of Silver
- Atomic Mass Average: 107.8682
- Boiling Point: 2436K 2163°C 3925°F
- Coefficient of lineal thermal expansion/K-1: 19.2E-6
- Conductivity
- Electrical: 0.63 106/cm Ω
Thermal: 4.29 W/cmK
- Electrical: 0.63 106/cm Ω
- Density: 10.5g/cc @ 300K
- Description:
- Very soft and malleable silver metal. Appearance and odor vary depending upon specific compound.
- Elastic Modulus:
- Bulk: 103.6/GPa
- Rigidity: 30.3/GPa
- Youngs: 82.7/GPa
- Enthalpy of Atomization: 284.5 kJ/mole @ 25°C
- Enthalpy of Fusion: 11.3 kJ/mole
- Enthalpy of Vaporization: 255.1 kJ/mole
- Flammablity Class: Non-combustible solid (except as dust)
- Freezing Point:see melting point
- Hardness Scale
- Brinell: 24.5 MN m-2
- Mohs: 2.5
- Vickers: 251 MN m-2
- Heat of Vaporization: 250.58kJ/mol
- Melting Point: 1234K 961°C 1762°F
- Molar Volume: 10.27 cm3/mole
- Optical Reflectivity: 97%
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 0.235J/gK
- Vapor Pressure = 0.342Pa@961°C
Regulatory / Health
- CAS Number
- 7440-22-4
- RTECS: VW3500000
- NFPA 704
- Health: 1
- Fire: 2
- Reactivity:
- Special Hazard:
- OSHAPermissible Exposure Limit (PEL)
- TWA: 0.01 mg/m3
- OSHA PEL Vacated 1989
- TWA: 0.01 mg/m3
- NIOSHRecommended Exposure Limit (REL)
- TWA: 0.01 mg/m3
- IDLH: 10 mg/m3
- Routes of Exposure: Inhalation; Ingestion; Skin and/or eye contact
- Target Organs: Nasal septum, skin, eyes
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: <0.003
- Bone/p.p.m: 0.01-0.44
- Liver/p.p.m: 0.005-0.25
- Muscle/p.p.m: 0.009-0.28
- Daily Dietary Intake: 0.0014-0.08 mg
- Total Mass In Avg. 70kg human: 2 mg
Who / Where / When / How
- Discoverer: Known to ancient civilization
- Discovery Location: Unknown
- Discovery Year: Unknown
- Name Origin:
- Latin argentum (silver). Silver from Anglo-Saxon seolfor for silver.
- Abundance of Silver:
- Earth's Crust/p.p.m.: 0.07
- Seawater/p.p.m.:
- Atlantic Suface: N/A
- Atlantic Deep: N/A
- Pacific Surface: 0.0000001
- Pacific Deep: 0.0000024
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): 7.1
- Sources of Silver:
- Found in ores called argentite (AgS), light ruby silver (Ag3 AsS3), dark ruby silver (Ag3SbS3) and brittle silver. Silver is often obtained as a by-product of refining other metals like copper and gold. World wide production is around 9950 tons per year. Primary mining areas are Mexico, Bolivia, Honduras, Canada, USA.
- Uses of Silver:
- Used in alloys for jewelry, in many compounds, photographic film and paper electronics, mirrors and batteries.
- Additional Notes:
Silver Menu
- Silver Page One
- Silver Page Two
- Silver Page Three
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Silver - Ag. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/25/2021
https://EnvironmentalChemistry.com/yogi/periodic/Ag.html
.

Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Ag.html'>echo Periodic Table of Elements: Silver - Ag (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Silver - Ag is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
PLEASE, if you like an article we published simply link to it on our website do not republish it.
Electrolysis
Electrolysis involves passing an electric current through either a molten salt or an ionic solution. The ions are 'forced' to undergo either oxidation (at the anode) or reduction (at the cathode). Most electrolysis problems are really stoichiometry problems with the addition of an amount of electric current. The quantities of substances produced or consumed by the electrolysis process is dependent upon the following:
- electric current measured in amperes or amps
- time measured in seconds
- the number of electrons required to produce or consume 1 mole of the substance
Three equations relate these quantities:
- amperes x time = Coulombs
- 96,485 coulombs = 1 Faraday
- 1 Faraday = 1 mole of electrons
amps & time ' no save> Coulombs ' no save> Faradays ' no save> moles of electrons
Use of these equations are illustrated in the following sections.
Calculating the Quantity of Substance Produced or Consumed
Silver Element Number Of Electrons
To determine the quantity of substance either produced or consumed during electrolysis given the time a known current flowed::
_Oxidation_States_for_First_Row_Transition_Metals.jpg?revision=1&size=bestfit&width=761&height=544)
- Write the balanced half-reactions involved.
- Calculate the number of moles of electrons that were transferred.
- Calculate the number of moles of substance that was produced/consumed at the electrode.
- Convert the moles of substance to desired units of measure.
- Write the half-reactions that take place at the anode and at the cathode.
How Many Electrons Does Silver Have
cathode (reduction) Fe3+ + 3 e- ' nosave> Fe(s)
- Calculate the number of moles of electrons.
- Calculate the moles of iron and of chlorine produced using the number of moles of electrons calculated and the stoichiometries from the balanced half-reactions. According to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of chlorine gas.
- Calculate the mass of iron using the molar mass and calculate the volume of chlorine gas using the ideal gas law (PV = nRT).
2' NOSAVE height=96 width=382>
Calculating the Time Required
To determine the quantity of time required to produce a known quantity of a substance given the amount of current that flowed:
- Find the quantity of substance produced/consumed in moles.
- Write the balanced half-reaction involved.
- Calculate the number of moles of electrons required.
- Convert the moles of electrons into coulombs.
- Calculate the time required.
- Convert the mass of Zn produced into moles using the molar mass of Zn.
- Write the half-reaction for the production of Zn at the cathode.
- Calculate the moles of e- required to produce the moles of Zn using the stoichiometry of the balanced half-reaction. According to the equation 2 moles of electrons will produce one mole of zinc.
- Convert the moles of electrons into coulombs of charge using Faraday's constant.
- Calculate the time using the current and the coulombs of charge.
Zn2+(aq) + 2 e- ' nosave> Zn(s)
Calculating the Current Required
To determine the amount of current necessary to produce a known quantity of substance in a given amount of time:
- Find the quantity of substance produced/or consumed in moles.
- Write the equation for the half-reaction taking place.
- Calculate the number of moles of electrons required.
- Convert the moles of electrons into coulombs of charge.
- Calculate the current required.
- Calculate the number of moles of H2. (Remember, at STP, 1 mole of any gas occupies 22.4 L.)
- Write the equation for the half-reaction that takes place.
- Calculate the number of moles of electrons. According to the stoichiometry of the equation, 4 mole of e- are required to produce 2 moles of hydrogen gas, or 2 moles of e-'s for every one mole of hydrogen gas.
- Convert the moles of electrons into coulombs of charge.
- Calculate the current required.
Hydrogen is produced during the reduction of water at the cathode. The equation for this half-reaction is:
4 e- + 4 H2O(l) ' nosave> 2 H2(g) + 4 OH-(aq)
